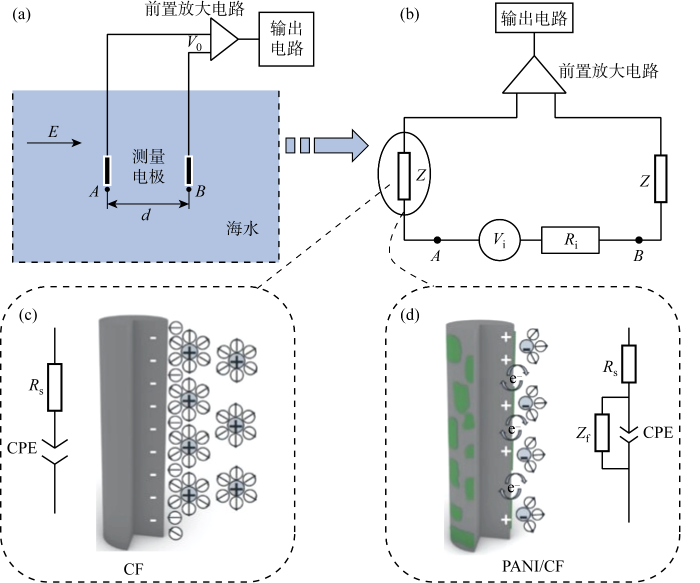
对水下电场的探测通过两电极间电压差变化反映[3-4].而在实海中,由于海水对电场存在衰减作用,只有低频的电场传输距离较远,所以主要检测的是低频电场.目前Ag/AgCl电极应用最广,但具有造价高、易见光分解、储存运输不便等缺点[5-6].相比较而言,碳纤维(Carbon Fiber, CF)电极依靠电极/海水界面双电层结构的变化实现对外电场的检测,虽然物理化学稳定性好并且成本更低,但是电极/海水界面双电层结构松散,电位稳定性差,电极自噪声高,因此难以实现对海中微弱电场信号的良好检测.CF表面引入极性基团有望改善表面双电层结构,提高该类电极的电场响应性能.如Liu等[4]在CF表面引入含氧、含氮基团,提高电极响应灵敏性;Zai等[7]将聚丙烯腈碳纤维(Polypropylene Cyanocarbon Fiber, PAN-CF)进行电化学氧化改性,改善电极自噪声和电场响应性能.
以PAN-CF为基体,利用电化学原位聚合法在其表面生成无机酸掺杂PANI导电薄膜,制备聚苯胺/碳纤维(PANI/CF)复合电极并首次用于海洋电场传感器.利用电化学性能和水下电场响应性能表征,验证PANI/CF复合电极用于海洋电场传感领域的可行性,并进一步探究不同无机酸掺杂对电极性能的影响机制.
1 实验部分
1.1 样品制备
实验使用未上浆PAN-CF,先后置于乙醇与丙酮体积比为1∶1的混合溶液和蒸馏水中超声清洗表面并烘干.
然后将PAN-CF制成简易电极接入电化学工作站中,对电极为铂片电极,参比电极为饱和甘汞电极.分别配制浓度为0.5 mol/L的盐酸、硫酸、磷酸溶液各500 mL,并加入质量分数为1%的苯胺单体制成电解液.设置聚合电流为0.1 A,聚合时间为10 min.聚合完成后再次清洗,得到不同酸掺杂的 PANI/CF 复合电极,并标记为PANI/CF-HCl、PANI/CF-H2SO4、PANI/CF-H3PO4.空白组为仅经过清洗的CF电极,标记为Blank.
PANI/CF电场传感器组装与配对.将制备好的PANI/CF复合电极烘干后封装至保护套,保护套由微孔材料制成,保证良好透水性的同时保护电极.测试电极电位稳定性测试,选取电位最为相近的两支电极编配成对,完成电场传感器的组装与配对.
1.2 CF表面特征与电化学测试
使用红外光谱和X射线光电子能谱(X-ray Photoelectron Spectroscopy, XPS)测试表征样品表面官能团和元素,扫描电子显微镜(Scanning Electron Microscope, SEM)观测样品表面形貌.应用循环伏安(Cyclic Voltammetry, CV)与电化学交流阻抗测试表征电化学性能.
1.3 电场性能测试
应用电极电位稳定性与电场信号频率响应测试表征电极电场性能.将电极置于盛有海水的水槽中,使用多通道信号记录仪(Agilent 34972A)采集电极电位.使用电场信号发射装置(Agilent 33509B)施加一定频率和振幅的正弦波电场信号模拟水下电场环境,再利用多通道信号记录仪记录电极响应情况.
采用拟合线性度表征配对电极响应的准确度,固定一定的发射信号频率,测量不同场强下的响应幅值,对最大响应幅值与施加信号峰值进行线性拟合,采用端基拟合直线方法计算配对电极在不同振幅下的响应线性误差,以最大线性误差作为其线性度,线性度越小就表示配对电极准确度越高.线性度计算公式如下:
式中:ΔLmax为测量值与拟合值之间的最大偏差;YF.S为满量程输出;k为拟合直线斜率;Xn-X1为满量程输出对应的x轴取值范围.
2 结果与分析
2.1 表面特征分析
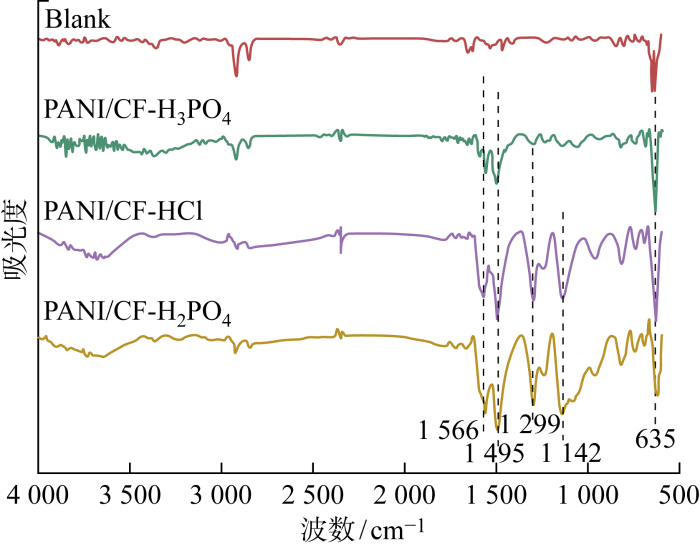
2.1.1 红外光谱分析
图1
表1 XPS定量分析得到的各元素相对含量
Tab.1
| 样品 | rC/% | rO/% | rN/% | rCl/% | rS/% | rP/% | nC/nN |
|---|---|---|---|---|---|---|---|
| PANI/CF-HCl | 68.38 | 16.22 | 12.77 | 2.62 | 5.35 | ||
| PANI/CF-H2SO4 | 64.73 | 25.36 | 8.20 | 1.70 | 7.89 | ||
| PANI/CF-H3PO4 | 65.50 | 22.33 | 11.44 | 0.72 | 5.73 |
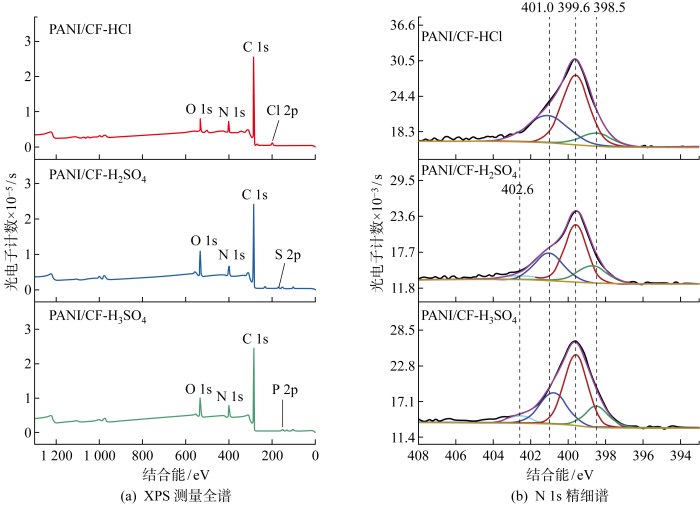
2.1.2 XPS分析
图2
图2
不同PANI/CF复合电极在3.5% NaCl溶液中1 mV/s扫速下的CV曲线
Fig.2
CV curves of different PANI/CF composite electrodes in 3.5% NaCl solution at a sweeping rate of 1 mV/s
PANI的通式为[(—B—NH—B—NH—)y(—B—N=Q=N—)1-y]x, 其中,B和Q分别表示苯型和醌型的C6H4环.通过N 1s分峰可进一步得到不同化学状态氮原子的相对含量.附图2(b)为N 1s分峰结果,其中389.5 eV为醌型环中的亚胺氮(—N=)[13],399.6 eV为苯型环中的胺氮(—NH—)[14],401.1 eV与402.6 eV为带正电荷的N+(氧化胺或质子化亚胺)[15-16].每个样品中不同化学状态下氮的比例由N 1s分峰确定并列于表2.从表中可以看出H2SO4掺杂的 PANI-N=/-NH—最大,表明PANI链中约有30%的醌式环和70%的苯式环;HCl掺杂的PANI-N=/-NH—最小,约有20%的醌式环和80%的苯式环.由N+/N可知,各类酸的掺杂水平基本一致,在32%~34%.
表2 精细谱拟合产生的不同氮基团对N 1s光电子谱的贡献
Tab.2
| 样品 | 贡献率/% | 贡献值 | |||
|---|---|---|---|---|---|
| —N= | —NH— | N+ | N+/N | —N=/—NH— | |
| 398.5 eV | 399.6 eV | 401.1 eV 402.6 eV | |||
| PANI/CF-HCl | 11.91 | 55.55 | 32.54 | 32.54 | 0.21 |
| PANI/CF-H2SO4 | 18.51 | 47.51 | 33.98 | 33.98 | 0.39 |
| PANI/CF-H3PO4 | 15.62 | 51.82 | 32.56 | 32.56 | 0.30 |
2.1.3 显微结构分析
2.2 电化学性能分析
2.2.1 循环伏安曲线分析
表3 空白碳纤维与PANI/CF电极材料的比电容值
Tab.3
| 电极 | 比电容值/(F·g-1) | 电容提升倍率 |
|---|---|---|
| Blank | 0.39 | 1.00 |
| PANI/CF-HCl | 55.17 | 141.46 |
| PANI/CF-H2SO4 | 65.37 | 167.62 |
| PANI/CF-H3PO4 | 31.85 | 81.67 |
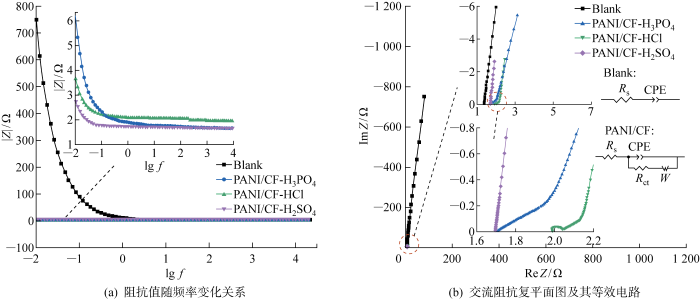
2.2.2 电化学阻抗分析
图3
表4 阻抗拟合结果
Tab.4
| 电极 | Rs/Ω | Rct/Ω | |Z|/Ω |
|---|---|---|---|
| Blank | 1.36 | 729 | |
| PANI/CF-HCl | 1.987 | 0.041 | 3.651 |
| PANI/CF-H2SO4 | 1.696 | 0.035 | 3.153 |
| PANI/CF-H3PO4 | 1.703 | 0.014 | 6.128 |
图3(b)所示的等效电路模型显示Blank电极为溶液电阻和双电层电容串联模型;PANI/CF复合电极增加了法拉第阻抗与双电层电容CPE并联,与CV测试结果相对应,这可能会增加电场传感器电极对微弱信号的响应灵敏度.此外复合电极液接电阻Rs有所增大,这是由于PANI膜电阻导致的.
2.3 电场性能
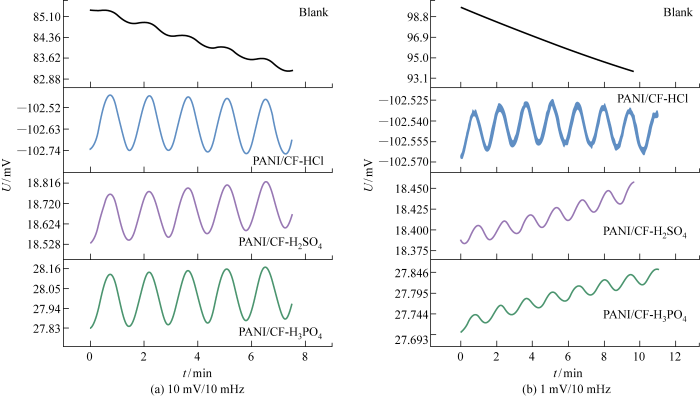
2.3.1 电极电位稳定性分析
图4为单个电极电位稳定性曲线(U-t)及配对电极电位差稳定性曲线(ΔU-t).在单个电极电位稳定性测试中,PANI/CF 复合电极电位曲线更为平稳.各电极稳定后 24 h 的电位波动(v)如表5所示,其中PANI/CF-H3PO4电极具有最小的电位波动(0.38 mV/d).在配对电极的电位差稳定测试中,Blank电极在刚入水后电位差波动较大,电极电位稳定需要一定的时间,不利于电场传感器电极的快速可用性.PANI/CF复合电极刚入水后的快速稳定性优于Blank电极,其中 PANI/CF-HCl 电极表现最佳,具有最小的平均电位差
图4
表5 电极电位漂移量
Tab.5
| 电极 | v/ (mV·d-1) | |
|---|---|---|
| Blank | 3.66 | 95.45 |
| PANI/CF-HCl | 1.77 | 34.96 |
| PANI/CF-H2SO4 | 4.52 | 143.51 |
| PANI/CF-H3PO4 | 0.38 | 78.66 |
2.3.2 电场信号响应分析
图5
图5
配对电场传感器电极对不同强度正弦波电场信号响应图谱
Fig.5
Response spectra of paired electrode electric field sensor to different intensity sine waves electric field signals
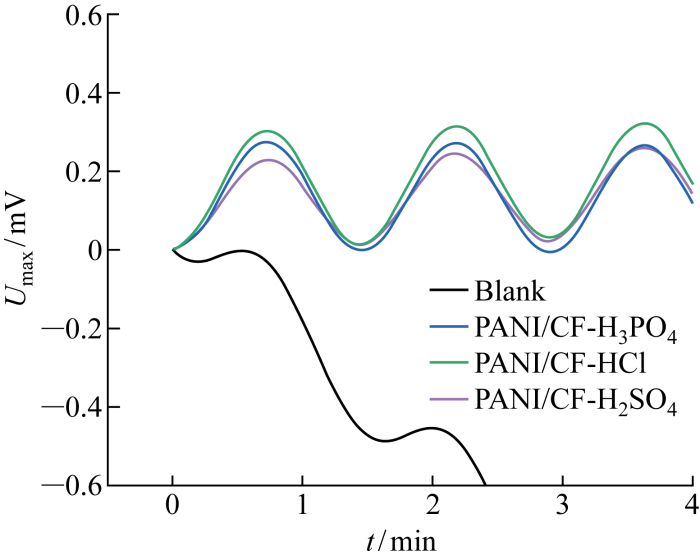
图6为配对电极对10 mV/10 mHz发射信号的最大响应幅值(Umax)比较结果,可以看出Blank电极漂移现象严重,与PANI/CF配对电极不具备可比性.在相同的发射信号强度下PANI/CF-HCl电极具有最大的响应幅值(0.30 V),比PANI/CF-H2SO4电极和PANI/CF-H3PO4电极幅值(0.23、0.27 V)分别提升约30%和11%.
图6
图6
10 mV/10 mHz条件下响应曲线的最大响应幅值比较
Fig.6
Comparison of maximum response amplitudes of response curves at 10 mV/10 mHz
由于PANI/CF-HCl电极表面PANI成膜均匀多孔(见图1(b)),PANI/CF-H2SO4电极表面 PANI 膜未完全覆盖碳纤维(见图1(c)),PANI/CF-H3PO4电极表面PANI存在团聚现象,厚度不均(见图1(d)),电极表面状态影响电极/海水界面形成的双电层结构;并且有研究利用多孔碳膜表面规律生成的疏水性和亲水性基团,实现液滴在材料表面的流动从而产生电势差,即水伏效应[20-21],所以电极表面成膜不均匀会有干扰信号,干扰电极对微弱电场信号的检测,成膜更均匀的PANI/CF-HCl电极受到的干扰最少.因而3种电极中PANI/CF-HCl电极电场响应性能最优,这也应与HCl掺杂电极具有较好的氧化还原可逆性有关.
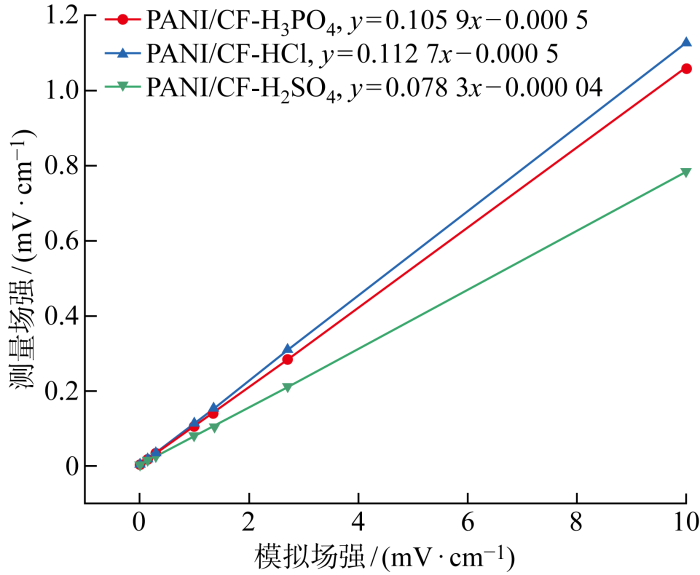
2.3.3 最大线性误差分析
为了研究配对电极响应水下电场信号的准确度,在固定频率0.1 Hz场源信号下,分别测量场幅为1、3.7、10、37、50、100、370 mV 时各类电极的最大响应幅值(见附录表2).通过E=U'/d换算出模拟场强与响应场强值,其中E为场强,U'为电势差,d为两个电极之间间距;对二者进行线性拟合得到各配对电极响应场强与相应场源信号模拟场强的关系(见图7),根据式(1)计算线性度,如表6所示.拟合直线斜率越大表明相同测试条件下该配对电极响应幅值越大,电极灵敏度越高.图中结果显示拟合直线斜率PANI/CF-H2SO4最小,PANI/CF-H3PO4次之,PANI/CF-HCl最大,说明PANI/CF-HCl电极具有最高的灵敏度.
图7
图7
配对电极响应场强与模拟场强关系
Fig.7
Relationship between response field strength of paired electrode and simulated field strength
表6 配对电极线性度拟合结果
Tab.6
| 配对电极 | k | γ/% |
|---|---|---|
| PANI/CF-HCl | 0.113 | 0.111 |
| PANI/CF-H2SO4 | 0.078 | 0.383 |
| PANI/CF-H3PO4 | 0.106 | 0.144 |
线性度γ代表配对电极对场源信号响应的准确度,γ越小表示配对电极响应准确度越高[22].计算结果表明,PANI/CF-HCl具有最小线性误差(0.111%).这说明PANI/CF-HCl既具有较高的响应灵敏度,又具有较好的响应准确度.
2.4 机理探究
图8
图8
电场传感器响应水下电场机理
Fig.8
Mechanism diagram of electric field sensor responding to underwater electric field signal
未经改性的CF电极在海水中无电化学反应发生,表面存在电荷积累,经过交流阻抗分析可等效为RC串联电路(见图8(c)).普通CF电极响应电场的电容结构由双电层构成,对电场的响应仅通过CF表面双电层结构的改变来完成.
PANI/CF电极表面有导电PANI膜生成,改变原先的纯电容性质,增加膜电容和膜电阻,其等效电路由原先的纯电容模型变为由电容部分和法拉第阻抗(Zf)并联的复合模型(见图8(d)),电场信号响应机制由原来的纯电容耦合改变为电容电阻共同作用的混合机制.PANI的引入使得CF表面含有极性较强的含氮基团,增强CF表面对溶液中带电粒子的吸附能力,形成的电容部分更稳定,由此PANI/CF电极电化学稳定性好、响应灵敏度高,这与其线性度、响应曲线、阻抗谱等结果相一致.并且从CV和阻抗结果可以看出,外电场变化时,PANI有离子掺杂和脱掺杂引起的氧化还原和电荷转移,会对复合电极电位和响应信号产生影响,因此PANI/CF电极的电场响应机理应是两者共同作用.
3 结论
(1) 应用无机酸掺杂PANI制备的新型海洋CF复合电极,具有不同于普通CF电极的电容电阻耦合响应机制和特征氧化还原峰,离子掺杂使其拥有更大比电容,较低的低频阻抗能够更好地响应水下微弱电场.新型电极制备工艺简单便捷,快速稳定性较好,便于运输和折叠,故该新型PANI/CF复合电极有望用于开发新一代低成本、高性能柔性电场探测传感器.
(2) 3种复合电极中,PANI/CF-HCl电极具有最优的综合电场响应性能,可较为稳定地响应 1 mV/10 mHz电场信号,具有低电位漂移量(1.77 mV/d)和低线性误差(0.111%).
(3) PANI/CF电极仍有一些不足,如快速稳定性弱于Ag/AgCl电极,实验室测试与实海测试有差异等.
(4) 未来可尝试在PANI/CF电极中掺杂本身带有极性基团的有机酸.还可研究PANI/CF-HCl电极在海水中Cl-掺杂机制与电场响应机制,并与Ag/AgCl电极响应机制比较,提高电极性能.
附录见本刊网络版(xuebao.sjtu.edu.cn/article/2024/1006-2467-58-03-391.shtml)
附录
图1
表1 PANI/CF电极红外特征峰位置
Tab.1
| 样品 | 波数/cm-1 | |||
|---|---|---|---|---|
 | 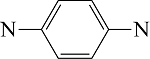 | C—N | C—H | |
| PANI/CF-HCl | 1567 | 1496 | 1299 | 1140 |
| PANI/CF-H2SO4 | 1566 | 1494 | 1299 | 1143 |
| PANI/CF-H3PO4 | 1557 | 1503 | 1299 | — |
注:—表示不适用.
图2
表2 PANI/CF电极实测幅值
Tab.2
| 配对电极 | 实测幅值 | ||||||
|---|---|---|---|---|---|---|---|
| 1 mV/0.1 Hz | 3.7 mV/0.1 Hz | 10 mV/0.1 Hz | 37 mV/0.1 Hz | 150 mV/0.1 Hz | 100 mV/0.1 Hz | 370 mV/0.1 Hz | |
| PANI/CF-HCl | 0.12 | 0.42 | 1.13 | 4.17 | 5.63 | 11.27 | 41.69 |
| PANI/CF-H2SO4 | 0.077 | 0.29 | 0.78 | 2.89 | 3.91 | 7.83 | 28.97 |
| PANI/CF-H3PO4 | 0.11 | 0.39 | 1.06 | 3.91 | 5.29 | 10.58 | 39.18 |
参考文献
舰船水下电磁场国外研究现状
[J].
Situation on underwater electromagnetic field researches of ships abroad
[J].
New advances in three-dimensional controlled-source electromagnetic inversion
[J].DOI:10.1111/gji.2008.172.issue-2 URL [本文引用: 1]
Ocean electric field tests of carbon fiber electrodes prepared by nitric acid oxidation
[J].
DOI:10.5755/j02.ms.20907
URL
[本文引用: 1]

The concentrated nitric acid oxidation was chosen as the surface treatments on carbon fibers in this paper. Treated carbon fibers were subjected to subsequent processing and forming, the electrodes applied in marine electric field detection were prepared. The electrochemical properties that related to practical application in marine electric field detection such as potential stability, polarization and cyclic voltammetry performance, the electrochemical impedance and the self-noise were characterized, thus an electrochemical evaluation system was formed to optimize the oxidation technology. Combining with the TGA and FTIR, the physical properties of carbon fibers were characterized and some oxidation laws were induced. The results indicate that the carbon fiber electrode prepared by oxidation 4 hours at 110 ℃ has the best electrochemical performance for detection.
Electrochemical and electric field response properties of highly sensitive electrodes based on carbon fiber with oxygen and nitrogen surface groups
[J].DOI:10.1109/JSEN.7361 URL [本文引用: 2]
Development and evaluation of an ultralow-noise sensor system for marine electric field measurements
[J].
Ag/AgCl电极海洋电场探测机理研究
[J].
Research on mechanism of marine electric field detection based on Ag/AgCl electrode
[J].
Oxidation modification of polyacrylonitrile-based carbon fiber and its electro-chemical performance as marine electrode for electric field test
[J].DOI:10.1007/s11802-020-4178-x [本文引用: 1]
聚苯胺及其高温碳化对海底微生物燃料电池阴极电化学性能影响
[J].
Effect of pyrolyzed polyaniline modified cathode on the electrochemical performance of marine sediment microbial fuel cells
[J].
Preparation by pulsed current electrochemical polymerisation and properties of ordered comb-shaped polyaniline/carbon fibres composites for flexible supercapacitor electrodes
[J].DOI:10.1080/00202967.2020.1728051 URL [本文引用: 1]
MXene-conducting polymer asymmetric pseudocapacitors
[J].DOI:10.1002/aenm.v9.7 URL [本文引用: 1]
The effects of acid leaching on porosity and surface functional groups of cocoa (Theobroma cacao)-shell based activated carbon
[J].
Comparative studies of solid-state synthesized polyaniline doped with inorganic acids
[J].
High-resolution XPS studies of electrochemically synthesized conducting polyaniline films
[J].DOI:10.1016/0379-6779(90)90240-L URL [本文引用: 1]
Comparative XPS surface study of polyaniline thin films
[J].DOI:10.1016/j.ssi.2008.08.004 URL [本文引用: 1]
Protonation of the amine nitrogens in emeraldine-evidence from X-ray photoelectron spectroscopy
[J].DOI:10.1016/0379-6779(92)90346-K URL [本文引用: 1]
X-ray photoelectron spectroscopy studies of some polyaniline-halogen complexes
[J].
Acid doping of polyaniline: Spectroscopic and electrochemical studies
[J].DOI:10.1021/jp991110z URL [本文引用: 1]
Electricity generation from water droplets via capillary infiltrating
[J].DOI:10.1016/j.nanoen.2018.02.061 URL [本文引用: 1]
Surface functional modification boosts the output of an evaporation-driven water flow nanogenerator
[J].DOI:10.1016/j.nanoen.2019.02.011 URL [本文引用: 1]
高性能碳纤维水下电场电极制备及其性能测量
[J].
DOI:10.3969/j.issn.1000-1093.2017.11.015
[本文引用: 1]

对T300碳纤维在445 ℃、465 ℃、485 ℃下进行表面热处理,以获得新型水下电场电极。利用X射线光电子能谱(XPS)分析、电化学工作站、自制电极响应装置和电极自噪声测量装置对其在NaCl溶液中的表面化学状态、电化学特性和探测性能进行了测量,讨论热处理温度对其表面基团含量、循环伏安特性、交换电流密度、响应性能和电极稳定时间的影响,分析了其电场探测机理。结果表明:热处理温度的提高可提高碳纤维表面基团CO和COOR含量,减小电极在NaCl溶液中的 电容效应,增加电极的交换电流密度,提高电极的线性性能和稳定速度。
Preparation and performance measurement of high performance underwater carbon fiber electric field electrode
[J].
DOI:10.3969/j.issn.1000-1093.2017.11.015
[本文引用: 1]

A novel underwater electric field electrode is prepared with T300 carbon fiber heat-treated at 445 ℃, 465 ℃and 485 ℃. The surface chemical state, electrochemical performance and detection performance of electric field electrode in NaCl solution are measured using X-ray photoelectron spectroscopy (XPS), electrochemical workstation and self-made noise measurement system. The impact of heat treatment on surface group content, cyclic voltammetry (CV) characteristics, exchange current density, response performance and electrode settling time is discussed. The detection mechanism of underwater carbon fiber detection electrode is analyzed. The group CO and COOR content of carbon fiber electrode increase as the heat treatment temperature increases; the heat treatment reduces the capacitance effect of electrode, increases its exchange current density, and improve its linear performance and settling speed. Key
Interfacial enhancement of carbon fiber composites by poly (amido amine) functionalization
[J].