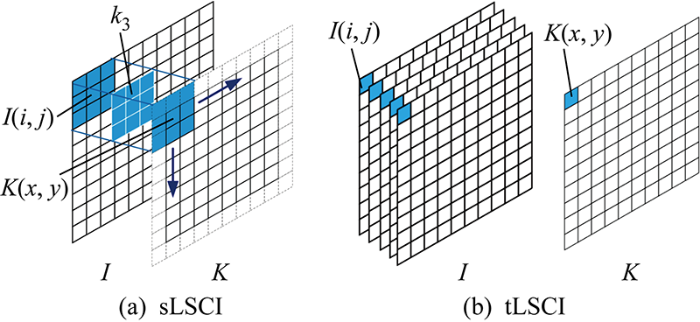
激光照射粗糙表面或高度散射介质会散射出具备不同散射角和光程的光束,它们在空间中某一点汇聚时会发生干涉,形成激光散斑,用成像设备观测到明暗相间的图案称作散斑图像.散斑现象在激光、超声波等相干波应用中十分常见,散斑可以当作检测材料形变的标记[1].激光散斑图像会因血液等散射介质运动而模糊,其模糊程度取决于流动快慢,因而可以借助激光散斑图像模糊程度进行血液流动速度的无接触测量.
高灰度级照相机能增加sLSCI稳定性[14],高分辨率能有效弥补sLSCI造成的空间分辨率降低问题,然而高分辨、高灰度级图像需要更长的计算时间,导致实时监测中延迟时间更长.使用硬件辅助计算,如现场可编程逻辑门阵列(FPGA),可以实现在空间分辨率由1 024像素×1 024像素降至256像素×256像素条件下提高处理速度与图像质量[15],但分辨率降低可能导致散斑与像素大小比值不符合奈奎斯特准则[16],加速的结果可能无法监测细小血管.sLSCI的标准差函数不能表示为线性运算的组合,sLSCI中相互独立的标准差计算循环耗时最多.为解决上述问题,提出通过简化sLSCI中标准差计算的近似计算及并行计算方法,有效缩短sLSCI耗时,从而达到高分辨、高灰度级图像临床实时监测血液流动要求,同时通过对比分析不同计算方法的LSCI结果,得到血液实时监测最佳方案.
1 激光散斑成像sLSCI方法
1.1 激光散斑衬比度计算
激光散斑成像衬比度K模型[17]为
式中:β为光学系统系数;T为照相机曝光时间;g1(τ)为电场自相关函数.通常认为g1(τ)=
图1
式中:x-N≤i≤x+N;y-N≤j≤y+N;(i, j)为计算核中的像素在散斑图I上的坐标.
1.2 sLSCI近似计算
近似计算方法简化了标准差,解决了式(2)中标准差计算无法线性计算的问题,避免了相互独立循环的计算.假设将计算核k2N+1内各点I(i, j)与中心点
1.3 sLSCI并行计算
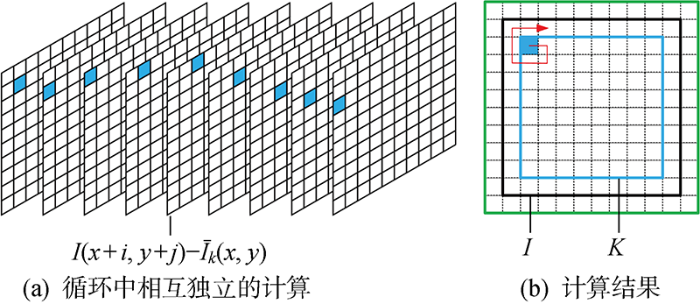
式(2)中,每次计算K(x, y)如图1(a)所示移动核k,以 2 048像素×2 048 像素图片计算核k3为例,一张图片需要 2 046×2 046 次循环,单循环9次计算.并行计算一次可以执行多个命令的算法,扩大求解规模来求解问题,但是CPU并行计算大量简单循环,进程调用会消耗大量时间.因此,处理一张图片只使用9次循环,单循环 2 046×2 046次计算可以减少循环次数,提高计算速度.
图2
考虑到每次计算结果都独立,利用式(2)进行并行计算,同时计算δI(i, j),可以减少单张sLSCI图片生成时间.在此基础上利用Python编写实时并行框架的激光散斑成像监测程序,使用开源OpenCV解决工业照相机因驱动程序导致丢帧的问题.
1.4 信噪比量化
信噪比 (SNR)是衡量一幅图像信号与噪声的比值.一般来说,成像质量越好,SNR越大.本文定义信噪比为
式中:
2 LSCI血流实验
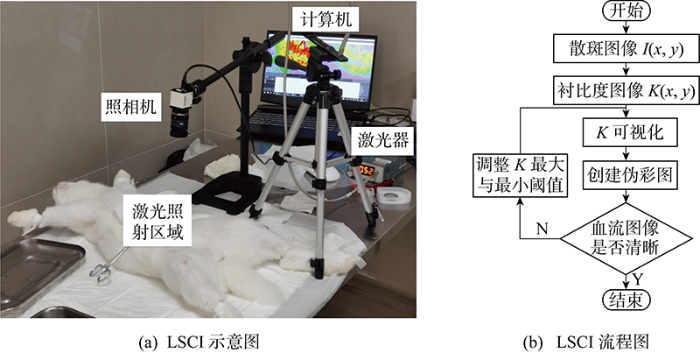
根据LSCI原理,结合临床应用中仪器与监测目标的最小距离等要求,设计并搭建LSCI系统原理样机.LSCI系统主要由3部分组成:激光器、成像系统以及计算机.激光的波长为785 nm,功率为60 mW.肠系膜分布着密集的毛细血管,适合用来检验血流监测效果,因此实验监测对象为兔子肠系膜.实验使用8位NS1044BU照相机(NET GmbH Company, Germany),分辨率为752像素×480像素(约36万),60帧/s与16位PCO.panda 4.2照相机(PCO Company, Germany),2 048像素×2 048像素(约420万),44帧/s拍摄.散斑图像均使用Python编写的Windows系统程序处理.使用伪彩图凸显流动信息会使不同计算方法的LSCI背景颜色差异过大,不利于比较,因此所有LSCI均使用灰度图.
兔子肠系膜血管实验在上海市第六人民医院完成,实验符合动物实验伦理要求.实验示意图与成像流程如图3所示.
图3
图3
LSCI实验示意图与LSCI流程图
Fig.3
Experimental schematic diagram and flow chart of LSCI
3 LSCI血流实验结果
3.1 低分辨率照相机成像
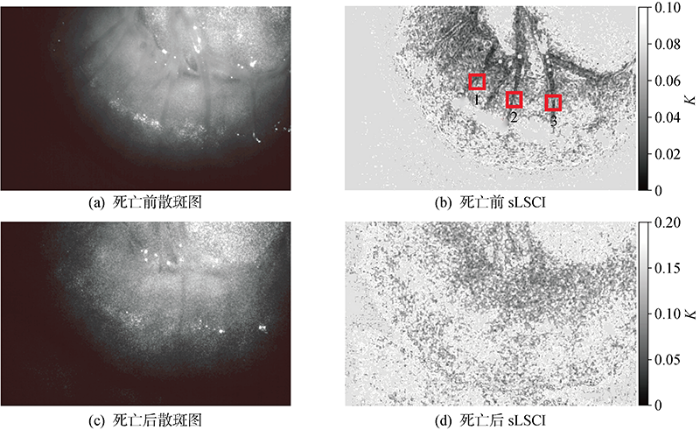
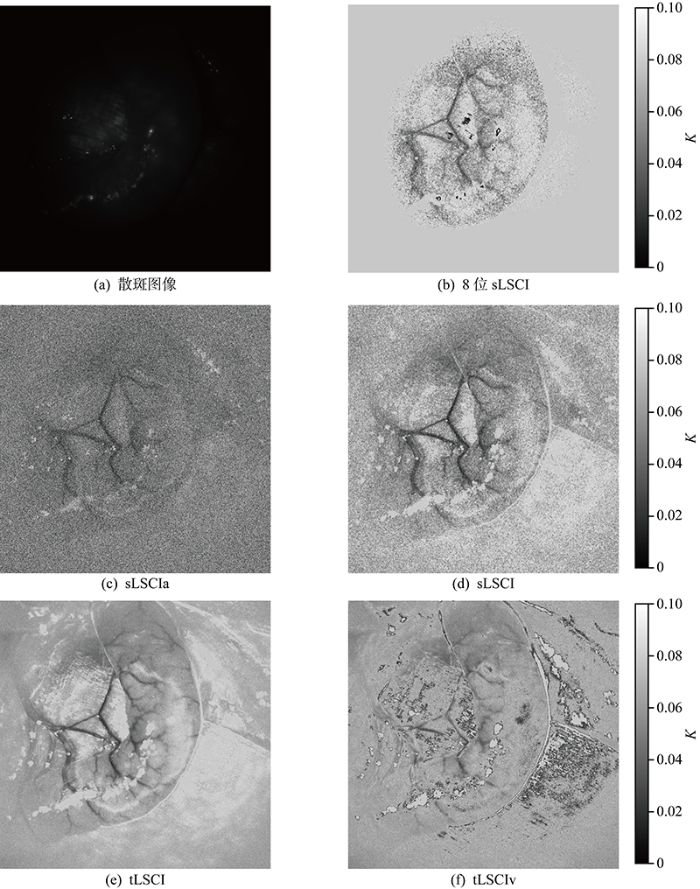
首先使用8位像素深度照相机验证LSCI技术能监测血液流动.死亡前后兔子肠系膜血管流动散斑图及sLSCI如图4所示.从图中可以看出,死亡前后兔子肠系膜血管区域的衬比度有明显差异,本研究开发的LSCI技术可以监测动物血液流动.实验中sLSCI的可视化结果灰度值表示K值大小,灰度值越小,图像颜色越深,代表血液流动越快.
图4
表1 兔子死亡前后肠系膜血管各感兴趣区间K的平均值与信噪比
Tab.1
| ROI编号 | RSN,l | RSN,d | ||
|---|---|---|---|---|
| ROI1 | 0.030 9 | 0.161 8 | 5.207 8 | 2.527 2 |
| ROI2 | 0.029 9 | 0.141 8 | 4.104 3 | 3.694 0 |
| ROI3 | 0.035 9 | 0.122 5 | 4.459 8 | 2.441 9 |
由表可知,兔子死亡前散斑图像的衬比度K明显小于死亡后散斑图像衬比度K,通过LSCI可以准确判断血管内是否有血液流动.
3.2 高灰度级照相机成像
受限于8位照相机低分辨率与16位照相机搭配镜头的最小对焦距离,将高分辨16位散斑图片转换成8位散斑图像,8位散斑图的计算结果记为8位sLSCI,其余LSCI均使用16位散斑图片.在照相机44fps拍摄的100张图像中,选择连续的20张,计算结果记为tLSCI,为理想状态的处理结果;实际处理中,图片的读取时间、目标与激光的波动都会减弱图片之间的相关性,因此在100张中均匀间隔选择20张,处理结果记为tLSCIv.为考虑扰动的tLSCI处理结果,使用k5计算sLSCI,sLSCI近似计算结果记为sLSCIa.不同计算方法处理得到的兔子肠系膜血管流动的可视化衬比度图片如图5所示.
图5
图5
16位2 048像素×2 048像素散斑图片不同计算方法LSCI
Fig.5
Different computational methods LSCI for 16-bit 2 048×2 048 pixels speckle images
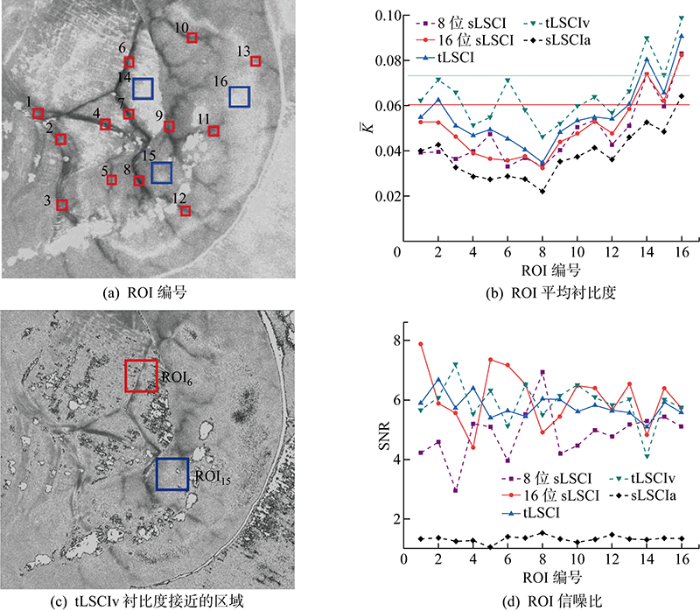
如图5所示,实验中激光照射在图像中心区域,实验中流动区域K均小于0.1,因此选择[0,0.1]区间凸显流动信息.图5(a)为处理前的原始图像,无法判断是否有血液流动,图5~图5(f)均能得到直观的血液流动信息.对比图5(b)与图5(d)可知,8位sLSCI只能得到光照中心区域的流动信息,16位sLSCI则可以得到更大区域的流动信息.图5(c)中sLSCIa血流与静态组织区域的对比度低于图5(d)中sLSCI,图5(e)中tLSCI血流与静态组织区域的对比度高于图5(f)中的tLSCIv.最后,选取16个ROI对比分析不同方法的成像质量,记作ROIi, i=1, …, 16;其中,13个红色区域为血管,3个蓝色区域为静态组织,如图6(a)所示.
图6
图6
不同计算方法的ROI的
Fig.6
表2
不同方法下各感兴趣区间
Tab.2
| 参数 | 取值 | ||||
|---|---|---|---|---|---|
| 8位sLSCI | 16位sLSCI | tLSCI | tLSCIv | sLSCIa | |
| 0.910 4 | 0.970 7 | 1.000 0 | 0.915 4 | 0.915 4 | |
| SNR的平均值 | 4.861 9 | 6.037 1 | 5.781 4 | 5.902 8 | 1.318 8 |
综上可知,tLSCIv的ROI6灰度与ROI15接近,且该区域成像SNR高,直观判断下该区域没有血液流动,与实际矛盾,易导致LSCI观察者误判.对比sLSCI,如图6(b)中红色直线所示,在光照中心区域质量优于tLSCIv.
通过对比8位与16位LSCI的SNR和对比度的结果,发现高灰度级照相机在成像质量上优于低灰度级照相机.对比16位图片不同方法LSCI的SNR与
3.3 并行计算效率
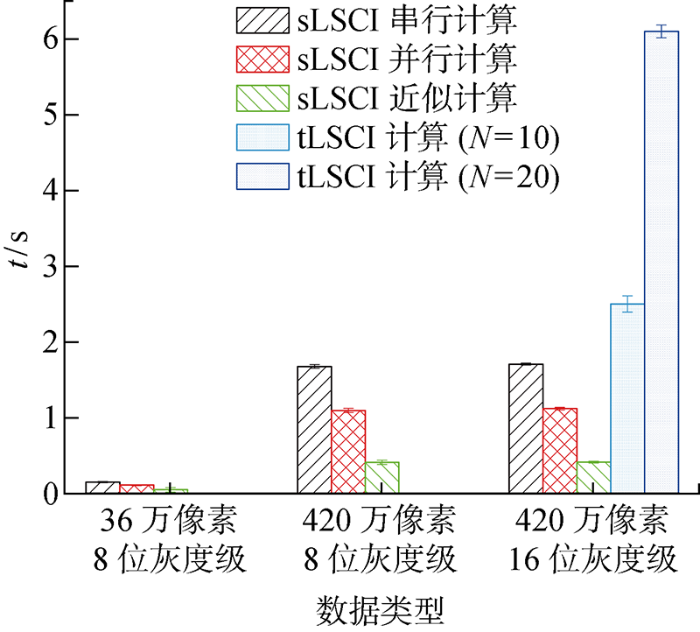
LSCI图像处理并行计算使用CPU进行实时计算,CPU为Intel(R)Core(TM) i5-9300H CPU@2.40 GHz.记录LSCI图像串行、并行处理及近似算法处理100张图片的平均消耗时间,如图7所示.
图7
由图7可知,实时监测程序记录100张tLSCI计算(N=20),平均每张tLSCI时间为6 s;sLSCI图像处理并行计算能有效加速图像处理速度,计算时间约为串行的2/3;sLSCI近似计算时间约为串行sLSCI的1/4;同等分辨率下,8位图像计算稍快于16位图像;420万像素图像计算时间明显大于36万像素图片计算时间.因此可以认为空间分辨率及空间统计标准差的计算是制约sLSCI计算时间的主要原因.
4 结语
提出一种高灰度级高分辨率血流LSCI技术的并行计算处理方法.通过兔子肠系膜血管成像发现,高分辨率下散斑图像光照中心区域sLSCI能达到理想tLCSI(N=20)的成像效果.实际应用中,sLSCI避免了tLSCI因外界干扰和序列中各图像间较大延迟时间造成的图像质量下降.近似计算sLSCIa的计算时间虽然是串行计算时间sLSCI的1/4,但信噪比与对比度明显低于sLSCI.基于Python并行计算的sLSCI相对于串行计算的sLSCI,计算时间消耗减少1/3.实验表明,高灰度级高分辨率空间激光散斑并行计算图像满足临床实时监测需求.
参考文献
Effects of speckle/pixel size ratio on temporal and spatial speckle-contrast analysis of dynamic scattering systems: Implications for measurements of blood-flow dynamics
[J].DOI:10.1364/BOE.4.001883 URL [本文引用: 1]
Speckle-visibility spectroscopy: A tool to study time-varying dynamics
[J].DOI:10.1063/1.2037987 URL [本文引用: 1]
基于局部特征点检测与匹配的微悬臂梁变形受力测量方法
[J].
A method for deformation and force measurement of micro-cantilever based on local feature point detecting and matching
[J].
Flow visualization by means of single-exposure speckle photography
[J].DOI:10.1016/0030-4018(81)90428-4 URL [本文引用: 1]
Comparison of indocyanine green angiography and laser speckle contrast imaging for the assessment of vasculature perfusion
[J].
DOI:10.1227/NEU.0b013e31826adf88
PMID:22843129
[本文引用: 1]

Assessment of the vasculature is critical for overall success in cranial vascular neurological surgery procedures. Although several methods of monitoring cortical perfusion intraoperatively are available, not all are appropriate or convenient in a surgical environment. Recently, 2 optical methods of care have emerged that are able to obtain high spatial resolution images with easily implemented instrumentation: indocyanine green (ICG) angiography and laser speckle contrast imaging (LSCI).To evaluate the usefulness of ICG and LSCI in measuring vessel perfusion.An experimental setup was developed that simultaneously collects measurements of ICG fluorescence and LSCI in a rodent model. A 785-nm laser diode was used for both excitation of the ICG dye and the LSCI illumination. A photothrombotic clot model was used to occlude specific vessels within the field of view to enable comparison of the 2 methods for monitoring vessel perfusion.The induced blood flow change demonstrated that ICG is an excellent method for visualizing the volume and type of vessel at a single point in time; however, it is not always an accurate representation of blood flow. In contrast, LSCI provides a continuous and accurate measurement of blood flow changes without the need of an external contrast agent.These 2 methods should be used together to obtain a complete understanding of tissue perfusion.
On the equivalence and differences between laser Doppler flowmetry and laser speckle contrast analysis
[J].DOI:10.1117/1.JBO.21.12.126018 URL [本文引用: 1]
Intraoperative assessment of parathyroid viability using laser speckle contrast imaging
[J].
DOI:10.1038/s41598-017-14941-5
PMID:29093531
[本文引用: 1]

Post-surgical hypoparathyroidism and hypocalcemia are known to occur after nearly 50% of all thyroid surgeries as a result of accidental disruption of blood supply to healthy parathyroid glands, which are responsible for regulating calcium. However, there are currently no clinical methods for accurately identifying compromised glands and the surgeon relies on visual assessment alone to determine if any gland(s) should be excised and auto-transplanted. Here, we present Laser Speckle Contrast Imaging (LSCI) for real-time assessment of parathyroid viability. Taking an experienced surgeon's visual assessment as the gold standard, LSCI can be used to distinguish between well vascularized (n = 32) and compromised (n = 27) parathyroid glands during thyroid surgery with an accuracy of 91.5%. Ability to detect vascular compromise with LSCI was validated in parathyroidectomies. Results showed that this technique is able to detect parathyroid gland devascularization before it is visually apparent to the surgeon. Measurements can be performed in real-time and without the need to turn off operating room lights. LSCI shows promise as a real-time, contrast-free, objective method for helping reduce hypoparathyroidism after thyroid surgery.
Development of an imaging device for label-free parathyroid gland identification and vascularity assessment
[J].
Clinical applications of laser speckle contrast imaging: A review
[J].
DOI:10.1117/1.JBO.24.8.080901
PMID:31385481
[本文引用: 1]

When a biological tissue is illuminated with coherent light, an interference pattern will be formed at the detector, the so-called speckle pattern. Laser speckle contrast imaging (LSCI) is a technique based on the dynamic change in this backscattered light as a result of interaction with red blood cells. It can be used to visualize perfusion in various tissues and, even though this technique has been extensively described in the literature, the actual clinical implementation lags behind. We provide an overview of LSCI as a tool to image tissue perfusion. We present a brief introduction to the theory, review clinical studies from various medical fields, and discuss current limitations impeding clinical acceptance.
激光散斑衬比血流成像技术研究进展
[J].
Laser speckle contrast imaging on in vivo blood flow: A review
[J].
Capillary-blood-flow monitoring using laser speckle contrast analysis (LASCA): Improving the dynamic range
[J].
Temporal statistical analysis of laser speckle images and its application to retinal blood-flow imaging
[J].
DOI:10.1364/oe.16.010214
PMID:18607429
[本文引用: 1]

Temporal-statistical analysis of laser-speckle image (TS-LSI) preserves the original spatial resolution, in contrast to conventional spatial-statistical analysis (SS-LSI). Concerns have been raised regarding the temporal independency of TS-LSI signals and its insensitivity toward stationary-speckle contamination. Our results from flow phantoms and in vivo rat retinas demonstrated that the TS-LSI signals are temporally statistically independent and TS-LSI minimizes stationary-speckle contamination. The latter is because the stationary speckle is "non-random" and thus non-ergodic where the temporal average of stationary speckle needs not equal its spatial ensemble average. TS-LSI detects blood flow in smaller blood vessels and is less susceptible to stationary-speckle artifacts.
High resolution cerebral blood flow imaging by registered laser speckle contrast analysis
[J].
DOI:10.1109/TBME.2009.2037434
PMID:20142159
[本文引用: 1]

Laser speckle imaging (LSI) has been widely used for in vivo detecting cerebral blood flow (CBF) under various physiological and pathological conditions. So far, nearly all literature on in vivo LSI does not consider the influence of disturbances due to respiration and/or heart beating of animals. In this paper, we analyze how such physiologic motions affect the spatial resolution of the conventional laser speckle contrast analysis (LASCA). We propose a registered laser speckle contrast analysis (rLASCA) method which first registers raw speckle images with a 3 x 3 convolution kernel, normalized correlation metric and cubic B-spline interpolator, and then constructs the contrast image for CBF. rLASCA not only significantly improves the distinguishability of small vessels, but also efficiently suppresses the noises induced by respiration and/or heart beating. In an application of imaging the angiogenesis of rat's brain tumor, rLASCA outperformed the conventional LASCA in providing a much higher resolution for new small vessels. As a processing method for LSI, rLASCA can be directly applied to other LSI experiments where the disturbances from different sources (like respiration, heart beating) exist.
Manhattan distance-based adaptive 3D transform-domain collaborative filtering for laser speckle imaging of blood flow
[J].
DOI:10.1109/TMI.2019.2896007
PMID:30714912
[本文引用: 1]

Laser speckle contrast imaging (LSCI) is a full-field, noncontact imaging technology for mapping blood flow with high spatio-temporal resolution, in which the speckle contrast can be estimated either in spatial domain or temporal domain. Temporal LSCI (tLSCI) provides higher spatial resolution than spatial domain does. However, when the number of sampling frames is limited, it is difficult to obtain accurate blood flow velocity owing to the significant statistical noise. The widely used spatially averaged tLSCI (savg-tLSCI) usually requires a large number of sampling frames to obtain acceptable denoising performance. Here, based on the nonlocal filtering strategy of block-matching and three-dimensional transform-domain collaborative filtering (BM3D), Manhattan distance-based adaptive BM3D (MD-ABM3D) is proposed to effectively manage the complicated inhomogeneous noise in tLSCI image and improve the signal-to-noise ratio. Manhattan distance improves the accuracy of the block matching in strong noise, and the adaptive algorithm adapts to the inhomogeneous noise and estimates suitable parameters for improved denoising. MD-ABM3D improves 4.91 dB in peak signal-to-noise ratio relative to savg-tLSCI. It achieves stability for denoising tLSCI image with different temporal windows. The image-quality evaluation of MD-ABM3D for tLSCI (t = 20 frames) equals that of savg-tLSCI (t = 60 frames). It achieves high signal-to-noise ratio with a reduced number of sampling frames. A reduced number of sampling frames are more practical for biomedical applications. It also offers higher temporal resolution and less disturbance from the motion of the moving object.
Sensitivity, noise and quantitative model of laser speckle contrast imaging
[D].
Effect of signal intensity and camera quantization on laser speckle contrast analysis
[J].
DOI:10.1364/BOE.4.000089
PMID:23304650
[本文引用: 1]

Laser speckle contrast analysis (LASCA) is limited to being a qualitative method for the measurement of blood flow and tissue perfusion as it is sensitive to the measurement configuration. The signal intensity is one of the parameters that can affect the contrast values due to the quantization of the signals by the camera and analog-to-digital converter (ADC). In this paper we deduce the theoretical relationship between signal intensity and contrast values based on the probability density function (PDF) of the speckle pattern and simplify it to a rational function. A simple method to correct this contrast error is suggested. The experimental results demonstrate that this relationship can effectively compensate the bias in contrast values induced by the quantized signal intensity and correct for bias induced by signal intensity variations across the field of view.
A 15.6 frames per second 1-megapixel multiple exposure laser speckle contrast imaging setup
[J].